Medical devices
The term medical device is used to name any instrument, device, equipment, material or product intended to be used for medical purposes.
A medical device can be in external contact with the patient or it can be partially or totally implanted.
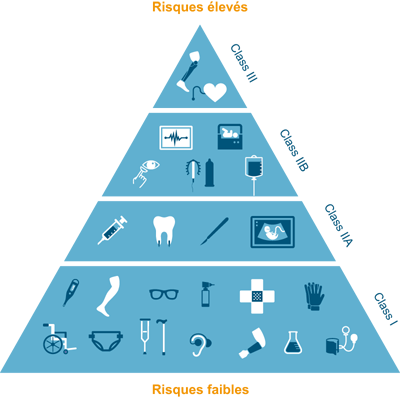
This is why there are different classes of medical devices depending on the risk they present to the health of the patient:
1) Low risk: glasses, crutches, ...
2) Moderate / measured risk: contact lenses, ultrasound devices, dental crowns, etc.
3) High / significant risk: lens disinfection products, condoms, etc.
4) Highest risk: implants, prostheses, ...

 EN
EN
 FR
FR